Lipid-based drug delivery systems (LBDDS) have been studied and developed since the 1960s. The main objective is to develop formulations that increase the bioavailability of drugs while reducing undesirable side effects. In 2020, this technology received a lot of attention due to its application in SARS-CoV-2 vaccination. In this first part of our LBDDS two-parter, we will focus on the structure and composition of lipid-based drug delivery systems and the different types of LBDDS. In addition to their application in mRNA vaccines, there are other possible uses for LBDDS, some of which we would like to introduce here.
These topics await you:
2) Which LBDD systems are available?
3) What components can LBDD systems be composed of?
What are LBDD systems?
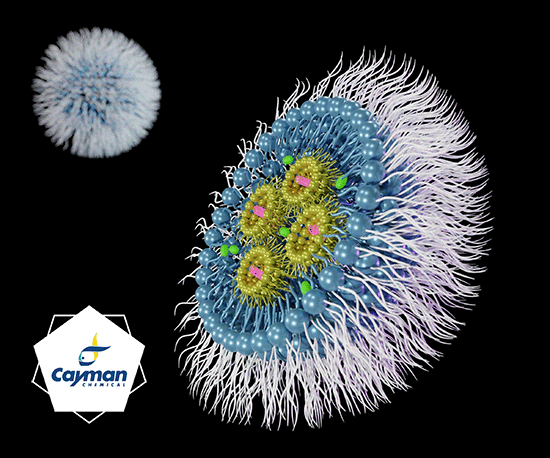
In 1964, the research group around Alec Douglas Bangham discovered the so-called liposome [1]. Liposomes are vesicles consisting of a lipid bilayer (e.g. phospholipids or fatty acids) and enclosing an aqueous phase inside (Fig. 1). The lipids are amphiphilic, meaning that the molecules have a lipophilic and a hydrophilic part. Within the lipid bilayer, the hydrophilic portions each face outward and the lipophilic portions face inward, allowing an aqueous phase to exist inside the liposome (Fig. 1). If the lipid structure of the liposome is reversed (when the lipophilic portions point outward), it is a so-called reverse-phase liposome, in which a lipophilic phase can be introduced inside [2]. The liposome represents the first commercially used LBDD system. Examples of approved drugs using liposomes as a drug delivery system include:
- Epaxal: Hepatitis A vaccine
- Influvac: influenza virus vaccine
- Amphotericin B: antifungal agent against systemic fungal infections
- Verteporfin: age-related macular degeneration, myopia
- Perflutren: contrast agent for ultrasound examination

Figure 1: Schematic representation of the liposome (source: Cayman Chemical). The envelope of liposomes consists of a lipid bilayer (e.g. phospholipids). The lipids have a lipophilic and a hydrophilic part. In classical liposomes, the lipophilic parts point to the inside of the lipid bilayer and the hydrophilic parts point to the outside, so that an aqueous phase can exist in the core of the liposome. Here, hydrophilic drugs can be encapsulated, whereas hydrophobic drugs can be packaged inside the lipid shell.
LBDD systems are highly versatile and allow for the delivery of diverse, bioactive molecules (such as small molecule inhibitors and vaccine components) into target cells and tissues. LBDDS have several advantages over conventional delivery methods. These include improved stability, higher bioavailability and better distribution of the drug. Poorly water-soluble drugs, in particular, pose challenges during delivery, which causes problems for the development of such drugs. Lipid-based drug delivery systems therefore lend themselves especially well to the administration of such agents. The delivery of drugs packaged via LBDD systems can generally be oral, parenteral, ocular, intranasal, and also dermal/transdermal. However, oral drug administration is generally preferred as it is both easiest and most cost-effective for the patient, especially with regard to the treatment of chronic diseases [3]. In addition to "the classic" liposome, a variety of other, different packaging units have been developed in the field of LBDD systems to meet different needs.
Which LBDD systems are available?
LBDD systems are generally differentiated according to their structure and the chemical nature of their core. Depending on their structure, they are suitable for the transport of different substances (Fig. 2).
1. Lipid nanoparticles (LNPs) consist of a lipid shell surrounding an inner core of reverse micelles. Oligonucleotides, such as siRNA, mRNA and plasmid DNA (pDNA), are encapsulated in these micelles.
2. Liposomes, mentioned earlier, consist of one or more lipid bilayers and an aqueous core. They are differentiated according to their lamellarity and size. Depending on the orientation of the lipid bilayer, liposomes can be used for the delivery of hydrophobic and/or hydrophilic small molecules (see also Fig. 1).
3. Solid lipid nanoparticles (SLNs) are composed of a surfactant shell surrounding a core matrix of solid lipids. They are used for the encapsulation of hydrophobic and/or hydrophilic active ingredients.
4. Nanostructured lipid carriers (NLCs) are also built up from a surfactant shell surrounding a core matrix of solid and liquid lipids. They are used for the encapsulation of hydrophobic and/or hydrophilic substances.
5. Micelles are a self-assembly of lipid monolayers in aqueous solutions. They have a hydrophobic core with the phospholipid tails facing inward. They can be used for encapsulation of small hydrophobic charges.
6. Reverse micelles are an inverted structure compared to traditional micelles. They form a hydrophilic core with the phospholipid tails facing outward. They can be used for encapsulation of small hydrophilic cargoes, such as oligonucleotides in LNPs.
Figure 2: Overview of different LBDD systems (source: Cayman Chemical).
What components can LBDD systems be composed of?
Lipid nanoparticles (LNPs) are mostly composed of cationic lipids, glycerophospholipids, sterols and PEG-associated lipids. These PEG-associated lipids contain the encapsulated oligonucleotides in an aqueous phase. Other LBDD systems often contain similar structural elements, but also surfactants, for example. The choice of structural lipids determines the behavior and properties of an LBDD particle, and therefore the selection of the right components is crucial in the formulation. For example, neutral phospholipids contribute to the membrane fusion efficiency of LBDD particles. Anionic lipids are often incorporated into neutral LBDD systems, especially to pack small molecules. They prevent aggregation of the small particles during storage. Sterols, such as cholesterol, usually make up 20-50% of LBDD formulations. They are used to fill defects in lipid membrane packing and ensure structural integrity [2]. In addition, they also help in membrane fusion of LNPs and target cell. If the envelope of the LBDD formulation is equipped with PEG-associated lipids, this can prevent the LBDD particles from mistakenly binding to serum proteins and being taken up and degraded by the mononuclear phagocyte system (MPS). Uptake by the MPS is an obstacle to LBDDS application and can be circumvented using such PEG-associated lipids. To encapsulate oligonucleotides in LNPs, cationic lipids are primarily used. Specially ionizable cationic lipids have been developed for this purpose, which do not produce unwanted cytotoxicity as is the case with normal cationic lipids.
LBDD systems offer many possibilities to deliver drugs effectively and with fewer side effects. Depending on the charge, different particles can be considered, which can be individually formulated and modified. Our partner Cayman Chemical offers high-quality lipid components that can be used to build a lipid particle as research tools, as well LNP Exploration Kits and Custom Lipid Synthesis to support preclinical research and drug development programs. You can find all LBDD products from Cayman by clicking on the link below.
Click here: All LBDD Products from Cayman Chemical
If you are interested in more details about LBDD systems, check out Cayman‘s new Guide to Lipid Nanoparticle Formulation. Also, stay tuned for the second part of our LBDDS series on our blog, in which you will learn more about the history of LNP as well as its medical applications, up to its use in the SARS-CoV-2 vaccination!
Sources:
[1] https://flexikon.doccheck.com/de/Liposom
[2] An Introduction to Lipid Nanoparticle Formulation: Basic Concepts & Preparation Procedures, Article from 2022-02-23, Christy R. Cuthbertson, Ph.D. and Melissa A. Parsey, Ph.D. - Technical Writers, Cayman Chemical
[3] Lipid-Based Drug Delivery Systems, Hina Shrestha, Rajni Bala, and Sandeep Arora, Journal of Pharmaceutics, Volume 2014, Article ID 801820
Fig. 1 and 2 from Cayman Chemical's https://www.caymanchem.com/news/intro-to-lipid-nanoparticle-formulation