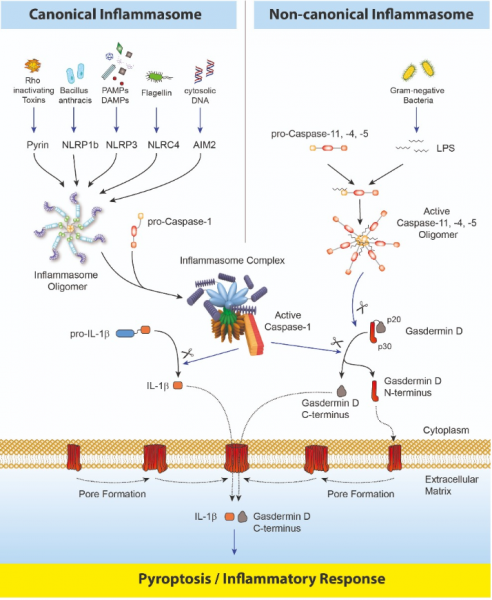
Inflammasomes are multimeric protein complexes that comprise a sensor (e.g. NLRP3), an adaptor (ASC/Pycard) and a protease (pro-caspase-1).1 An inflammasome assembles in response to a diverse range of pathogen-associated or danger-associated molecular patterns (PAMPs or DAMPs), or perturbations in cytoplasmic homeostasis (term 'homeostasis-altering molecular processes' (HAMPs)).2 The inflammasome platform leads to activation of caspase-1, which further induces maturation of interleukin-1β and -18 (IL-1β and IL-18) through proteolytic cleavage of pro-IL-1β and pro-IL-18. Activated caspase-1, and also the recently characterized caspase-11 non-canonical inflammasome pathway, cleave the newly discovered intracellular protein Gasdermin D.3,4 The Gasdermin family members contain N-terminal domains that are capable of forming membrane pores, whereas the C-terminal domains of Gasdermins function as inhibitors of such cytolysis through intramolecular domain association.
Caspase-1 or -11 cleavage of Gasdermin D is required for regulation of Pyroptosis: upon caspase-1/11 cleavage of the Gasdermin N- and C-domain linker, the cleaved N-terminal fragment of Gasdermin D oligomerizes and forms pores on the host cell membrane,5 leading to a cell death called pyroptosis and further activation of inflammasomes by triggering K+ efflux.6 Gasdermin D forming pores regulate the non-conventional secretion of cytokines such as IL-1β, in response to cytosolic LPS and other activators of the inflammasome.7 Neutrophil extrusion of neutrophil extracellular traps (NETs) and concomitant cell death (NETosis), a particular neutrophil defense against pathogens, are dependent on Gasdermin D cleavage by caspase-11.8 Gasdermin D-mediated pyroptosis is regulated at the level of lipid peroxidation9 and seems to be a key effector in the LPS-induced lethal sepsis.10
Caspase-8, an upstream activator of caspase-3, controls apoptotic cell death and prevents RIPK3–MLKL-dependent necroptosis. Caspase-8, activated by the Yersinia effector protein YopJ, also triggers Gasdermin D processing and cell death with different Yersinia species.11
After formation of the pore at the cellular membrane by Gasdermin D N-terminal fragment, the role and fate of the C-terminus fragment of Gasdermin D is still unclear. Using the Gasdermin D (mouse) ELISA Kit (Prod. No. AG-45B-0011-KI01), that detects the C-terminal part of Gasdermin D (as well as the full-length protein), a signal is detected in the supernatant of cells dying by pyroptosis, suggesting that the C-terminal fragment is released from cells, either by chance due to the presence of a pore or for a specific task not yet clear.
Gasdermin D (mouse) ELISA Kit
The Gasdermin D (mouse) ELISA Kit detects full-length and cleaved mouse Gasdermin D (C-terminus) in cell culture supernatants and cell extracts. It does not cross-react with human gasdermin D.

- Detection Type: Colorimetric
- Assay Type: Sandwich
- Sample Type: Cell Culture Supernatant, Cell Lysate
- Sensitivity: 14 pg/ml
- Range: 15.6 to 1000 pg/ml
References
- The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta: F. Martinon, et al.; Mol. Cell 10, 417 (2002)
- Homeostasis-altering molecular processes as mechanisms of inflammasome activation: A. Liston & S.L. Masters; Nat. Rev. Immunol. 17, 208 (2017)
- Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling: N. Kayagaki, et al.; Nature 526, 666 (2015)
- Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death: S. Feng, et al.; J. Mol. Biol. 430, 3068 (2018)
- Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores: X. Liu, et al.; Nature 535, 153 (2016)
- Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis: I. Banerjee, et al.; Immunity 49, 413 (2018)
- The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages: C.L. Evavold, et al.; Immunity 48, 35 (2018)
- Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps: K.W. Chen, et al.; Sci. Immunol. 3, 26 (2018)
- Lipid Peroxidation Drives Gasdermin D-mediated Pyroptosis in Lethal Polymicrobial Sepsis: R. Kang, et al.; Cell Host Microbe 24, 97 (2018)
- Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis: J.K. Rathkey, et al.; Sci. Immunol. 3, 26 (2018)
- Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death: P. Orning, et al.; Science (Epub ahead of print) 26 (2018)