A fluorescent probe is a fluorophore designed to localize within a specific region of a biological specimen or to respond to a specific stimulus. Fluorescent probes enable researchers to detect particular components of complex biomolecular assemblies (including live cells) with exquisite sensitivity and selectivity. Reactive fluorescent dyes are widely used to modify amino acids, peptides, proteins (in particular, antibodies), oligonucleotides, nucleic acids, carbohydrates and other biological molecules. AAT Bioquest provides a full spectrum of fluorophores for labeling biopolymers and derivatizing low molecular weight molecules. Among the reactive dyes, amine-reactive dyes are most often used to prepare various bioconjugates for immunochemistry, histochemistry, fluorescence in situ hybridization (FISH), cell tracing, receptor binding and other biological applications since amino groups are either abundant or easily introduced into biomolecules. In general, thiol-reactive reagents are frequently used to develop probes for investigating some particular protein structures and functions. Additionally, some amine-containing fluorescent reagents are also used to modify biomolecules, in particular, to label glycoproteins. In general, the preferred bioconjugates should have high fluorescence quantum yields and retain the biological activities of the unlabeled biomolecules. It is quite critical to properly control the degree of substitution (DOS) when conducting a conjugation of biopolymers. A high degree of labeling may significantly decrease the water solubility and binding affinity/specificity of the target biomolecules. Although conjugating dyes to biomolecules is usually easy, preparing the optimal conjugate may require extensive experimentation. Fortunately there are some excellent publications that may provide you some important guidelines.
Amine-Reactive Fluorescent Dyes
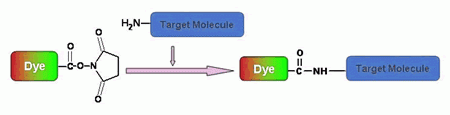
Amine-reactive fluorescent probes are widely used to modify peptides, proteins, oligonucleotides, nucleic acids, ligands and other biomolecules. Amine-reactive dyes are most often used to prepare bioconjugates for immunochemistry, fluorescence in situ hybridization (FISH), cell tracing, receptor labeling and fluorescent analog cytochemistry. In these applications, the stability of the chemical bond between the amine-reactive dye and biomolecule is particularly important because the fluorescent conjugates are often subjected to rigorous incubation, hybridization and washing steps. A number of fluorescent amino-reactive dyes have been developed to label various biomolecules, and the resultant conjugates are widely used in biological applications. Three major classes of amine-reactive fluorescent reagents are currently used to label biopolymers: succinimidyl esters (SE), isothiocyanates, and sulfonyl chlorides. AAT Bioquest offers all the popular amine-reactive fluorescent dyes for peptide/protein labelings, nucleotide modifications and microarray applications. Although FITC (fluorescein isothiocyanate), one of the most popular fluorescent labeling dyes, is predominantly used for preparing a variety of fluorescent bioconjugates, the low conjugation efficiency of FITC and the short life time of its conjugates are still troublesome for some critical biological applications. We strongly recommend that you choose succinimidyl esters for labeling needs if other conditions and factors are equivalent.
Fluorescent Dye Carboxylic Acids and Their Succinimidyl Esters
Succinimidyl esters are proven to be the best reagents for amine modifications because the amide bonds formed are essentially identical to, and as stable as the natural peptide bonds. These reagents are generally stable and show good reactivity and selectivity with aliphatic amines. There are a few factors that should be considered when SE compounds are used for conjugation reaction:
-
Solvents: For the most part, reactive dyes are hydrophobic molecules and should be dissolved in anhydrous dimethylformamide (DMF) or dimethylsulfoxide (DMSO)
-
Reaction pH: The labeling reactions of amines with succinimidyl esters are strongly pH dependent. Amine-reactive reagents react with non-protonated aliphatic amine groups, including the terminal amines of proteins and the ε-amino groups of lysines. Thus amine acylation reactions are usually carried out at pH > 7.5. Protein modifications by succinimidyl esters can typically be done at pH 7.5-8.5, whereas isothiocyanates may require a pH between 9.0 and 10.0 for optimal conjugations.
-
Reaction Buffers: Buffers that contain free amines such as Tris and glycine and thiol compounds must be avoided when using an amine-reactive reagent. Ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation must also be removed (such as viadinlysis) before performing dye conjugations.
-
Reaction Temperature: Most conjugations are done at room temperature. However, either elevated or reduced temperature may be required for a particular labeling reaction.
Fluorescent Dye Sulfonyl Chlorides

Sulfonyl chlorides are highly reactive. These reagents are unstable in water, especially at the higher pH required for reaction with aliphatic amines. Molecular modifications by sulfonyl chlorides need to be carefully carried out preferably at low temperature. Sulfonyl chlorides can also react with phenols (including tyrosine), aliphatic alcohols (including polysaccharides), thiols (such as cysteine) and imidazoles (such as histidine), but these reactions are not common in proteins or in aqueous solution. There are a few factors that need to be considered when SC compounds are used for conjugation reaction:
-
Solvents: SC dyes are generally hydrophobic molecules and should be dissolved in anhydrous dimethylformamide (DMF). Sulfonyl chlorides are unstable in dimethylsulfoxide (DMSO) and should never be used in this solvent.
-
Reaction pH: The labeling reactions of amines with SC reagents are strongly pH dependent. SC reagents react with non-protonated amine groups. On the other hand, the sulfonylation reagents tend to hydrolyze in the presence of water, with the rate increasing as the pH increases. Thus sulfonylation-based conjugations may require a pH 9.0-10.0 for optimal conjugations. In general, sulfonylation-based conjugations have much lower yields than the succinimidyl ester-based conjugations.
-
Reaction Buffers: Buffers that contain free amines such as Tris and glycine must be avoided when using an amine-reactive reagent. Ammonium sulfate and ammonium must be removed before performing dye conjugations. High concentrations of nucleophilic thiol compounds should also be avoided because they may react with the labeling reagent to form unstable intermediates that could destroy the reactive dye.
-
Reaction Temperature: Most SC conjugations are done at room temperature. However, reduced temperature may be required for a particular SC labeling reaction.
Fluorescent Dye Isothiocyanates

Isothiocyanates form thioureas upon reaction with amines. It is proven that some thiourea products (in particular, the conjugates from α-amino acids/peptides/proteins) are much less stable than the conjugates that are prepared from the corresponding succinimidyl esters. It has been reported that antibody conjugates prepared from fluorescein isothiocyanates deteriorate over time. We strongly recommend that you use succinimidyl esters for your conjugations whenever possible. There are a few factors that need to be considered when isothiocyanate compounds are used for conjugation reaction:
-
Solvents: For the most part, reactive dyes are hydrophobic molecules and should be dissolved either in anhydrous dimethylformamide (DMF) or in dimethylsulfoxide (DMSO).
-
Reaction pH: The labeling reactions of amines with isothiocyanates are strongly pH dependent. Isothiocynate reagents react with non-protonated aliphatic amine groups, including the terminal amines of proteins and the ε-amino groups of lysines. Protein modifications by isothiocyanates may require a pH 9.0-10.0 for optimal conjugations.
-
Reaction Buffers: Buffers that contain free amines such as Tris and glycine must be avoided when using an amine-reactive reagent. Ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation must also be removed before performing dye conjugations. High concentrations of nucleophilic thiol compounds should also be avoided because they may react with the labeling reagent to form unstable intermediates that could destroy the reactive dye.
-
Reaction Temperature: Most isothiocyanate conjugations are done at room temperature. However, either elevated or reduced temperature may be required for a particular labeling reaction.
Other Amine-Reactive Fluorescent Reagents
Electron-deficient aryl halides are effective amine-reactive labeling reagents for the preparation of various bioconjugates. AAT Bioquest offers fluorescamine, NBD chloride, NBD fluoride, AAT fluoride, SBD fluoride, 5- and 6-fluorescein dichlorotriazines. Due to their low selectivity, these dyes also react with thiol moieties besides amino groups. 5- and 6-fluorescein dichlorotriazines may even react with hydroxy compounds such as carbohydrates.
Non-fluorescent NBD chloride readily reacts with primary aliphatic amines (such as amino acids), generating bright yellow fluorescent amine adducts. NBD also reacts with thiols, although these adducts absorb and emit at shorter wavelengths and are less fluorescent than amine derivatives. NBD fluoride usually yields the same products as NBD chloride but is much more reactive. NBD fluoride may even react with hydroxy group under harsh conditions when amino or thiol groups are not available. The absorption and fluorescence emission spectra, quantum yields and extinction coefficients of NBD conjugates are all markedly dependent on the surrounding environment. Like the Dansyl dye adducts, the fluorescence quantum yield of NBD adducts of amines in water are very low (<0.01). NBD adducts of secondary amines are less fluorescent than that of primary amines while the adducts of aromatic amines with NBD are essentially non-fluorescent. There are a few factors that need to be considered when electron-deficient aryl halides are used for conjugation reaction.
-
Solvents: For the most part, electron-deficient aryl halides are hydrophobic molecules and should be dissolved in anhydrous dimethylformamide (DMF) or dimethylsulfoxide (DMSO).
-
Reaction pH: The labeling reactions of amines with electron-deficient aryl halides are strongly pH dependent. A pH of 7.5–9.5 is usually optimal for modifying lysine residues.
-
Reaction Buffers: Buffers that contain free amines such as Tris and glycine must be avoided when using an amine-reactive reagent. Ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation must also be removed before performing dye conjugations. High concentrations of nucleophilic thiol compounds should also be avoided because they readily react with the labeling reagent.
-
Reaction Temperature: Most conjugations are done at room temperature. However, either elevated or reduced temperature may be required for a particular labeling reaction.
Thiol-Reactive Fluorescent Dyes
Because free thiol (SH) groups, also called mercapto groups, are not present as abundantly as amino groups in most biopolymers such as proteins and nucleic acids, thiol-reactive reagents often provide a means of selectively modifying a protein at a defined site. Therefore thiol-reactive dyes are often used to prepare fluorescent peptides, proteins and oligonucleotides for probing biological structures, functions and interactions. Thiol-reactive dyes have been used to develop probes for analyzing the topography of proteins in biological membranes, determining distances within the protein or between the proteins and monitoring the changes in protein conformation using environment-sensitive probes.
There are many types of thiol-reactive dyes reported in the literature, including iodoacetamides, disulfides, maleimides, vinyl sulfones and various electron-deficient aryl halides and sulfonates. Iodoacetamides and maleimides are by far the most popular thiol-reactive moieties.
Fluorescent Dye Iodoacetamides (IA)

Iodoacetamides are one of the most popular thiol-reactive moieties for labeling biopolymers and small biomolecules. Iodoacetamides readily react with thiol moieties of biopolymers and small biomolecules to form thioether conjugates. The thioether bond formed is quite stable. Although iodoacetamides generally have good selectivity to thiol groups, they may react with histidine or potentially tyrosine under higher pH if free thiols are not readily available. The bioconjugation reactions of thiol-reactive probes can be quenched by the addition of cysteine, glutathione or mercaptosuccinic acid to the reaction mixture, forming highly water-soluble adducts that are easily removed by dialysis or gel filtration. There are quite a few factors that need to be considered when iodoacetamides are used for conjugation reaction:
-
Solvents: Most iodoacetamide dyes are hydrophobic molecules and should be dissolved in anhydrous dimethylformamide (DMF). Dimethyl sulfoxide (DMSO) should be avoided whenever possible since some particular iodoacetamides may be oxidized in DMSO at elevated temperature.
-
Reaction pH: The labeling reactions of thiol compounds with iodoacetamides are strongly pH dependent. Thiol-reactive reagents more readily react with thiol groups (such as cysteine and reduced glutathione) at higher pH. However, higher pH also increases the oxidative dimerization of thiol compounds. Thus thiol conjugations of iodoacetamides are often run in carbonate buffers with a pH ranging from 7.5 to 9.5. A pH of 8.5–9.5 is usually optimal for modifying cysteine residues.
-
Reaction Buffers: High concentrations of nucleophilic thiol compounds should also be avoided because they compete for the labeling reagent and, as a result, decrease conjugation yields. Buffers that contain free amines such as Tris and glycine should be avoided whenever possible since some iodoacetamides may also react with amines. Ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation should be removed before performing dye conjugations.
-
Reaction Temperature: Most conjugations are done at room temperature. However, either elevated or reduced temperature may be required for a particular labeling reaction.
-
Light Sensitivity: Iodo compounds are known to be very light-sensitive, especially in solution. Thus, we recommend the reactions of iodoacetamides with biomolecules be carried out under subdued light.
-
Avoiding Oxygen: Air oxidation of thiol compounds (to disulfides) is a major competing reaction for the iodoacetamide modifications of thiol componunds. It is recommended that air exposure of reaction solution should be minimized whenever possible.
Fluorescent Dye Maleimides

Maleimides and iodoacetamides are by far the most popular thiol-reactive moieties. Maleimides readily react with thiol moieties of biopolymers to form thioether conjugates even under neutral conditions. The thioether bond formed is quite stable. Maileimides are generally much less light-sensitive than iodoacetamides. The latter compounds are known to be very light liable, especially in solution.
Maleimides require conjugation conditions less stringent than those of iodoacetamides as described above. Unlike iodoacetamides, maleimides do not react with histidine and methionine under physiological conditions. For example, most conjugations can be done at room temperature at neutral pH. However, either elevated or reduced pH or temperature may be required for a particular labeling reaction.
Other Thiol-Reactive Fluorescent Dyes
NBD chloride was first introduced as a fluorogenic derivatization reagent for amines. It also reacts with thiols to form adducts that absorb and emit at shorter wavelengths. NBD and SBD compounds are widely used for fluorogenic thiol modifications of biopolymers. In general, NBD compounds are more often used for modifying amino groups and SBD compounds for selective modifications of thiol groups. The absorption and fluorescence emission spectra, quantum yields and extinction coefficients of NBD-thiol conjugates are dependent on the surrounding environment. SBD is an analog of NBD. SBD fluoride has been used for the derivatization of both amino and thiol groups. Quite a few excellent reviews have been published for the applications of SBD, NBD and SBD compounds. Most of NBD and SBD compounds react with both amino and thiol groups except that SBD-Cl has good selectivity to thiol groups. Additionally, NBD and SBD compounds are widely used for HPLC derivatizations.
Bromobimanes including monobromobimane and dibromobimane are another class of popular thiol-reactive fluorescent tags, and are widely used to detect various thiol-containing biomolecules such as glutathione in cells. The monobromobimanes are essentially non-fluorescent until they react with several low molecular weight thiols, including glutathione, N-acetylcysteine, mercaptopurine, peptides and plasma thiols, as well as with carboxylic acids. They are fluorogenic upon reacting with thiol-containing molecules. Monobromobimane is the most extensively used bimane derivative. These reagents are also useful for detecting the distribution of protein thiols in cells before and after chemical reduction of disulfides. Both monobromobimane and the more thiol-selective monochlorobimane have been extensively used for detecting glutathione in live cells. Monobromobimane can also be used to derivatize thiol-containing proteins prior to separation by isoelectric focusing without appreciably modifying the proteins electrophoretic mobility. Dibromobimane is an interesting crosslinking reagent for proteins because it is unlikely to fluoresce until both of its alkylating groups have reacted.
Carbonyl-Reactive (Amine-Containing) Fluorescent Dyes and Their Applications
Amine-containing dyes are widely used to modify water-soluble biopolymers (such as proteins) through the formation of Schiff Base or reductive amination. Among them, fluorescently labeled cadaverine and lysine derivatives and a variety of hydrazides have been predominantly used for modifying biomolecules. These dyes are used for modifications of carbohydrates, glycoproteins and nucleic acids that are first periodate-oxidized to introduce aldehydes and ketones into the biopolymers for subsequent reductive amination. The combination of periodate oxidation with reductive amination provides an effective way for site-selective modifications of biopolymers. For example, periodate oxidation of the 3-terminal ribose is reported to be one of the few methods of selectively modifying RNA. Periodate-oxidized ribonucleotides are converted to fluorescent nucleotide probes by reaction with fluorescent hydrazines and amines.
Amine-containing dyes are also used to modify biopolymers (such as proteins) using water-soluble carbodiimides (such as EDC) to convert the carboxy groups of the biopolymers into amide groups. Either NHS or NHSS may be used to improve the coupling efficiency of EDC-mediated protein–carboxylic acid conjugations. A large excess of the amine-containing dyes is usually used for EDC-mediated bioconjugations in concentrated protein solutions at low pH to reduce intra- and inter-protein coupling to lysine residues, a common side reaction.
The amine-containing dyes are also valuable building blocks in bioorganic and medicinal chemistry. We have used our amine-containing dyes to custom-synthesize many fluorescently labeled drugs, natural toxins and biological ligands.




![MCA [7-Methoxycoumarin-4-acetic acid] MCA [7-Methoxycoumarin-4-acetic acid]](https://www.biomol.com/media/image/a4/a2/1e/ABD-557_200x200.gif)

![6-JOE, SE [6-Carboxy-4,5-dichloro-2,7-dimethoxyfluorescein, succinimidyl ester] 6-JOE, SE [6-Carboxy-4,5-dichloro-2,7-dimethoxyfluorescein, succinimidyl ester]](https://www.biomol.com/media/image/5e/60/7f/ABD-203_200x200.gif)



![6-ROX [6-Carboxy-X-rhodamine] (Single isomer) 6-ROX [6-Carboxy-X-rhodamine] (Single isomer)](https://www.biomol.com/media/image/af/3a/bc/ABD-382_200x200.gif)

