Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| KMA-0100 | 1 kit | - | - |
6 - 10 business days* |
798.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
This kit includes: 96-well plate, endotoxin standard, chromogenic lysate (60 tests), LAL reagent... more
Product information "EndoAlert(TM) Endotoxin Plate Kit"
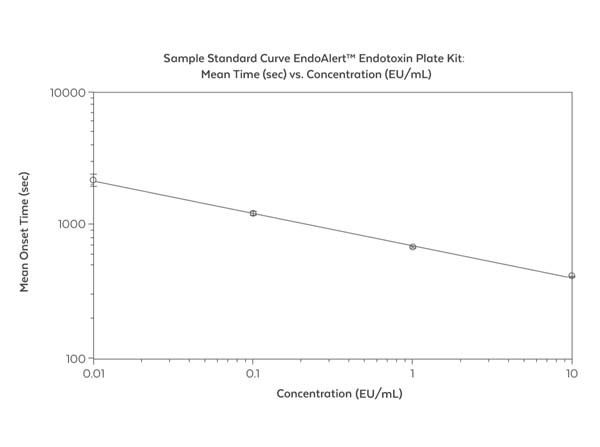
This kit includes: 96-well plate, endotoxin standard, chromogenic lysate (60 tests), LAL reagent water. See kit protocol for complete details. The EndoAlert(TM) Endotoxin Plate Kit provides a level of Endotoxin only in relation to the standard. It is NOT specific to the species of gram-negative bacteria which is the source of the Endotoxin in the sample. To increase accuracy of the test when the source of Endotoxin is known, use a purified Endotoxin from that species. The EndoAlert(TM) Endotoxin Plate Kit is a kinetic, colorimetric assay for the quantitative determination of bacterial Endotoxin in aqueous solutions. Endotoxin, a bacterial lipopolysaccharide, is one of the major cell wall components of most gram-negative bacteria. The EndoAlert(TM) Endotoxin Plate Kit detects low levels of Endotoxin and is therefore a useful tool to assess the integrity of biological and environmental samples. The detection ranges from 0.01 - 10 Endotoxin units (EU/mL). The EndoAlert(TM) Endotoxin Plate Kit is a quantitative version of the reaction first described by Levin and Bang in 1968. The test is based upon an enzymatic cascade where Endotoxin activates Factor C in Limulus Amebocyte Lysate (LAL) which in turn activates Factor B. Factor B activates Proclotting Enzyme which then activates Clotting Enzyme. A colorless synthetic peptide substrate is hydrolyzed by Clotting Enzyme to generate a yellow color which can be measured by a spectrophotometer at 405 nm. The degree of color resulting from the reaction is proportional to the amount of Endotoxin in the test sample and can be calculated using a standard curve.
| Keywords: | EndoAlert(TM) Endotoxin Plate Kit |
| Supplier: | Rockland Immunochemicals |
| Supplier-Nr: | KMA-0100 |
Properties
Database Information
Handling & Safety
| Storage: | +20°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed