Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Organism: Homo sapiens (Human). Source: E.coli. Expression Region: 26-760aa. Protein Length:... more
Product information "Prolyl endopeptidase FAP (FAP), partial, human, recombinant"
Organism: Homo sapiens (Human). Source: E.coli. Expression Region: 26-760aa. Protein Length: partial. Tag Info: N-terminal GST-tagged. Target Protein Sequence: LRPSRVHNSE ENTMRALTLK DILNGTFSYK TFFPNWISGQ EYLHQSADNN IVLYNIETGQ SYTILSNRTM KSVNASNYGL SPDRQFVYLE SDYSKLWRYS YTATYYIYDL SNGEFVRGNE LPRPIQYLCW SPVGSKLAYV YQNNIYLKQR PGDPPFQITF NGRENKIFNG IPDWVYEEEM LATKYALWWS PNGKFLAYAE FNDTDIPVIA YSYYGDEQYP RTINIPYPKA GAKNPVVRIF IIDTTYPAYV GPQEVPVPAM IASSDYYFSW LTWVTDERVC LQWLKRVQNV SVLSICDFRE DWQTWDCPKT QEHIEESRTG WAGGFFVSTP VFSYDAISYY KIFSDKDGYK HIHYIKDTVE NAIQITSGKW EAINIFRVTQ DSLFYSSNEF EEYPGRRNIY RISIGSYPPS KKCVTCHLRK ERCQYYTASF SDYAKYYALV CYGPGIPIST LHDGRTDQEI KILEENKELE NALKNIQLPK EEIKKLEVDE ITLWYKMILP PQFDRSKKYP LLIQVYGGPC SQSVRSVFAV NWISYLASKE GMVIALVDGR GTAFQGDKLL YAVYRKLGVY EVEDQITAVR KFIEMGFIDE KRIAIWGWSY GGYVSSLALA SGTGLFKCGI AVAPVSSWEY YASVYTERFM GLPTKDDNLE HYKNSTVMAR AEYFRNVDYL LIHGTADDNV HFQNSAQIAK ALVNAQVDFQ AMWYSDQNHG LSGLSTNHLY THMTHFLKQC FSLSD. Purity: Greater than 90% as determined by SDS-PAGE. Endotoxin: Not test. Biological Activity: n/a. Form: Liquid or Lyophilized powder. Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0. Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20 °C/-80 °C. Our default final concentration of glycerol is 50%. Customers could use it as reference. Storage: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20 °C/-80 °C. The shelf life of lyophilized form is 12 months at -20 °C/-80 °C. Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4 °C for up to one week. Relevance: Cell surface glycoprotein serine protease that participates in Extracellular domain matrix degradation and involved in many cellular processes including tissue rodeling, fibrosis, wound healing, inflammation and tumor growth. Both plasma mbrane and soluble forms exhibit post-proline cleaving endopeptidase activity, with a marked preference for Ala/Ser-Gly-Pro-Ser/Asn/Ala consensus sequences, on substrate such as alpha-2-antiplasmin SERPINF2 and SPRY2 . Degrade also gelatin, heat-denatured type I collagen, but not native collagen type I and IV, vibronectin, tenascin, laminin, fibronectin, fibrin or casein . Have also dipeptidyl peptidase activity, exhibiting the ability to hydrolyze the prolyl bond two residues from the N-terminus of synthetic dipeptide substrates provided that the penultimate residue is proline, with a preference for Ala-Pro, Ile-Pro, Gly-Pro, Arg-Pro and Pro-Pro . Natural neuropeptide hormones for dipeptidyl peptidase are the neuropeptide Y (NPY), peptide YY (PYY), substance P (TAC1) and brain natriuretic peptide 32 (NPPB) . The plasma mbrane form, in association with either DPP4, PLAUR or integrins, is involved in the pericellular proteolysis of the Extracellular domain matrix (ECM), and hence promotes cell adhesion, migration and invasion through the ECM. Plays a role in tissue rodeling during development and wound healing. Participates in the cell invasiveness towards the ECM in malignant melanoma cancers. Enhances tumor growth progression by increasing angiogenesis, collagen fiber degradation and apoptosis and by reducing antitumor response of the immune syst. Promotes glioma cell invasion through the brain parenchyma by degrading the proteoglycan brevican. Acts as a tumor suppressor in melanocytic cells through regulation of cell proliferation and survival in a serine protease activity-independent manner. Reference: Generation and annotation of the DNA sequences of human chromosomes 2 and 4.Hillier L.W., Graves T.A., Fulton R.S., Fulton L.A., Pepin K.H., Minx P., Wagner-McPherson C., Layman D., Wylie K., Sekhon M., Becker M.C., Fewell G.A., Delehaunty K.D., Miner T.L., Nash W.E., Kremitzki C., Oddy L., Du H. , Sun H., Bradshaw-Cordum H., Ali J., Carter J., Cordes M., Harris A., Isak A., van Brunt A., Nguyen C., Du F., Courtney L., Kalicki J., Ozersky P., Abbott S., Armstrong J., Belter E.A., Caruso L., Cedroni M., Cotton M., Davidson T., Desai A., Elliott G., Erb T., Fronick C., Gaige T., Haakenson W., Haglund K., Holmes A., Harkins R., Kim K., Kruchowski S.S., Strong C.M., Grewal N., Goyea E., Hou S., Levy A., Martinka S., Mead K., McLellan M.D., Meyer R., Randall-Maher J., Tomlinson C., Dauphin-Kohlberg S., Kozlowicz-Reilly A., Shah N., Swearengen-Shahid S., Snider J., Strong J.T., Thompson J., Yoakum M., Leonard S., Pearman C., Trani L., Radionenko M., Waligorski J.E., Wang C., Rock S.M., Tin-Wollam A.-M., Maupin R., Latreille P., Wendl M.C., Yang S.-P., Pohl C., Wallis J.W., Spieth J., Bieri T.A., Berkowicz N., Nelson J.O., Osborne J., Ding L., Meyer R., Sabo A., Shotland Y., Sinha P., Wohldmann P.E., Cook L.L., Hickenbotham M.T., Eldred J., Williams D., Jones T.A., She X., Ciccarelli F.D., Izaurralde E., Taylor J., Schmutz J., Myers R.M., Cox D.R., Huang X., McPherson J.D., Mardis E.R., Clifton S.W., Warren W.C., Chinwalla A.T., Eddy S.R., Marra M.A., Ovcharenko I., Furey T.S., Miller W., Eichler E.E., Bork P., Suyama M., Torrents D., Waterston R.H., Wilson R.K.Nature 434:724-731(2005). Function: Cell surface glycoprotein serine protease that participates in extracellular matrix degradation and involved in many cellular processes including tissue remodeling, fibrosis, wound healing, inflammation and tumor growth. Both plasma membrane and soluble forms exhibit post-proline cleaving endopeptidase activity, with a marked preference for Ala/Ser-Gly-Pro-Ser/Asn/Ala consensus sequences, on substrate such as alpha-2-antiplasmin SERPINF2 and SPRY2
| Keywords: | Recombinant Human Prolyl endopeptidase FAP (FAP), partial |
| Supplier: | Cusabio |
| Supplier-Nr: | EP008424HU |
Properties
| Application: | Activity not tested |
| Conjugate: | No |
| Host: | E.coli |
| Species reactivity: | human |
| MW: | 112 kD |
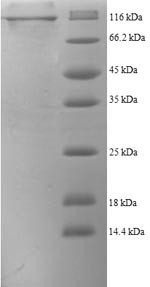
| Purity: | >90% (SDS-PAGE) |
Database Information
| KEGG ID : | K08674 | Matching products |
| UniProt ID : | Q12884 | Matching products |
| Gene ID : | GeneID 2191 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
You will get a certificate here
Viewed

