Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Organism: Homo sapiens (Human). Source: E.coli. Expression Region: 25-293aa. Protein Length:... more
Product information "Endoplasmic reticulum chaperone BiP (HSPA5), partial, human, recombinant"
Organism: Homo sapiens (Human). Source: E.coli. Expression Region: 25-293aa. Protein Length: Partial. Tag Info: N-terminal 6xHis-tagged. Target Protein Sequence: EDVGTVVGID LGTTYSCVGV FKNGRVEIIA NDQGNRITPS YVAFTPEGER LIGDAAKNQL TSNPENTVFD AKRLIGRTWN DPSVQQDIKF LPFKVVEKKT KPYIQVDIGG GQTKTFAPEE ISAMVLTKMK ETAEAYLGKK VTHAVVTVPA YFNDAQRQAT KDAGTIAGLN VMRIINEPTA AAIAYGLDKR EGEKNILVFD LGGGTFDVSL LTIDNGVFEV VATNGDTHLG GEDFDQRVME HFIKLYKKKT GKDVRKDNRA VQKLRREVE. Purity: Greater than 90% as determined by SDS-PAGE. Endotoxin: Not test. Biological Activity: n/a. Form: Liquid or Lyophilized powder. Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0. Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20 °C/-80 °C. Our default final concentration of glycerol is 50%. Customers could use it as reference. Storage: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20 °C/-80 °C. The shelf life of lyophilized form is 12 months at -20 °C/-80 °C. Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4 °C for up to one week. Relevance: Probably plays a role in facilitating the assbly of multimeric protein complexes inside the endoplasmic reticulum. Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10, probably to facilitate the release of DNAJC10 from its substrate. Reference: Chao C.C.K. Grp78 is involved in the quality control of the LDL-receptor.Hansen J.J., Nielsen M.N., Jorgensen M.M., Gregersen N., Bolund L. Sequence differences between human grp78/BiP isolated from HeLa cells and previously reported human sequences.Bermudez-Fajardo A., Llewellyn D.H., Campbell A.K., Errington R.R.NIEHS SNPs programDNA sequence and analysis of human chromosome 9.Humphray S.J., Oliver K., Hunt A.R., Plumb R.W., Loveland J.E., Howe K.L., Andrews T.D., Searle S., Hunt S.E., Scott C.E., Jones M.C., Ainscough R., Almeida J.P., Ambrose K.D., Ashwell R.I.S., Babbage A.K., Babbage S., Bagguley C.L. , Bailey J., Banerjee R., Barker D.J., Barlow K.F., Bates K., Beasley H., Beasley O., Bird C.P., Bray-Allen S., Brown A.J., Brown J.Y., Burford D., Burrill W., Burton J., Carder C., Carter N.P., Chapman J.C., Chen Y., Clarke G., Clark S.Y., Clee C.M., Clegg S., Collier R.E., Corby N., Crosier M., Cummings A.T., Davies J., Dhami P., Dunn M., Dutta I., Dyer L.W., Earthrowl M.E., Faulkner L., Fleming C.J., Frankish A., Frankland J.A., French L., Fricker D.G., Garner P., Garnett J., Ghori J., Gilbert J.G.R., Glison C., Grafham D.V., Gribble S., Griffiths C., Griffiths-Jones S., Grocock R., Guy J., Hall R.E., Hammond S., Harley J.L., Harrison E.S.I., Hart E.A., Heath P.D., Henderson C.D., Hopkins B.L., Howard P.J., Howden P.J., Huckle E., Johnson C., Johnson D., Joy A.A., Kay M., Keenan S., Kershaw J.K., Kimberley A.M., King A., Knights A., Laird G.K., Langford C., Lawlor S., Leongamornlert D.A., Leversha M., Lloyd C., Lloyd D.M., Lovell J., Martin S., Mashreghi-Mohammadi M., Matthews L., McLaren S., McLay K.E., McMurray A., Milne S., Nickerson T., Nisbett J., Nordsiek G., Pearce A.V., Peck A.I., Porter K.M., Pandian R., Pelan S., Phillimore B., Povey S., Ramsey Y., Rand V., Scharfe M., Sehra H.K., Shownkeen R., Sims S.K., Skuce C.D., Smith M., Steward C.A., Swarbreck D., Sycamore N., Tester J., Thorpe A., Tracey A., Tromans A., Thomas D.W., Wall M., Wallis J.M., West A.P., Whitehead S.L., Willey D.L., Williams S.A., Wilming L., Wray P.W., Young L., Ashurst J.L., Coulson A., Blocker H., Durbin R.M., Sulston J.E., Hubbard T., Jackson M.J., Bentley D.R., Beck S., Rogers J., Dunham I.Nature 429:369-374(2004). Function: Plays a role in facilitating the assembly of multimeric protein complexes inside the endoplasmic reticulum. Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10, probably to facilitate the release of DNAJC10 from its substrate (By similarity).
| Keywords: | BiP, GRP-78, HSP70 family protein 5, Binding-immunoglobulin protein, 78 kDa glucose-regulated protein, Endoplasmic reticulum chaperone BiP, Heat shock protein family A member 5, Heat shock protein 70 family protein 5, Recombinant Human Endoplasmic reticul |
| Supplier: | Cusabio |
| Supplier-Nr: | EP010827HU1 |
Properties
| Application: | Activity not tested |
| Conjugate: | No |
| Host: | E.coli |
| Species reactivity: | human |
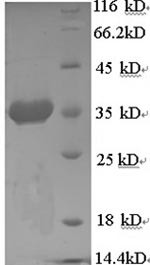
| MW: | 33.6 kD |
| Purity: | >90% (SDS-PAGE) |
Database Information
| KEGG ID : | K09490 | Matching products |
| UniProt ID : | P11021 | Matching products |
| Gene ID : | GeneID 3309 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
You will get a certificate here
Viewed

