Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| T5000.1 | 1 g | - | - |
3 - 19 business days* |
437.00€
|
||
| T5000.5 | 5 g | - | - |
3 - 19 business days* |
1,357.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
The lysine analog, thialysine, has been implicated in the mechanism for the inhibitory effect of... more
Product information "Thialysine (S-Aminoethyl-L-cysteine hydrochloride)"
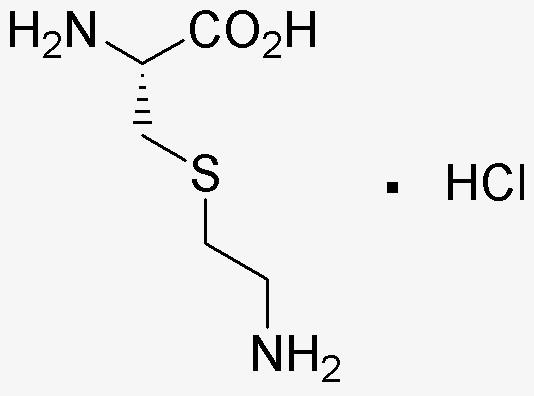
The lysine analog, thialysine, has been implicated in the mechanism for the inhibitory effect of the on human acute leukemia Jurkat T cells. When Jurkat T cells were treated with thialysine (0.32-2.5mM), apoptotic cell death along with several biochemical events such as mitochondrial cytochrome c release, caspase-9 activation, caspase-3 activation, degradation of poly (ADP-ribose) polymerase, and DNA fragmentation was induced in a dose- and time-dependent manner. However, these thialysine-induced apoptotic events were significantly abrogated by an ectopic expression of Bcl-xL, which is known to block mitochondrial cytochrome c release. Decylubiquinone, a mitochondrial permeability transition pore inhibitor, also suppressed thialysine-induced apoptotic events. Comparison of the thialysine-induced alterations in the cell cycle distribution between Jurkat T cells transfected with Bcl-xL gene (J/Bcl-xL) and Jurkat T cells transfected with vector (J/Neo) revealed that the apoptotic cells were mainly derived from the cells accumulated in S and G2/M phases following thialysine treatment. The interruption of cell cycle progression in the presence of thialysine was accompanied by a significant decline in the protein level of cdk4, cdk6, cdc2, cyclin A, cyclin B1, and cyclin E. These results demonstrate that the cytotoxic activity of thialysine toward Jurkat T cells is attributable to not only apoptotic cell death mediated by a mitochondria-dependent death signaling pathway, but also interruption of cell cycle progression by a massive down-regulation in the level of cdks and cyclins. Synonyms: S-(2-Aminoethyl)-L-cysteine Monohydrochloride, NSC 186915,, S-(2-Aminoethyl)-L-cysteine Hydrochloride,, S-(2-Aminoethyl)-L-cysteine Monohydrochloride,, S-(beta-Aminoethyl)-L-cysteine Hydrochloride, Thialysine Hydrochloride, CAS Number: 4099-35-8, Molecular Formula: C5H12N2O2S·HCl, Molecular Weight: 200.70, Appearance: Supplied as a light beige powder, Purity (TLC): ~99%, Solubility (H2O, 50mg/ml): Light tan, clear, Melting Point: 200-202°C , Specific Rotation (a,24 D): As reported (1% in water), Important Note: This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications without the expressed written authorization of United States Biological. Toxicity and Hazards: All products should be handled by qualified personnel only, trained in laboratory procedures.
| Supplier: | United States Biological |
| Supplier-Nr: | T5000 |
Properties
| Formula: | C5H12N2O2S?Cl |
Database Information
| CAS : | 4099-35-8| Matching products |
Handling & Safety
| Storage: | +4°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed

