Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Levonordefrin is the (-)-enantiomer of nordefrin, a synthetic derivative of norepinephrine... more
Product information "Levonordefrin"
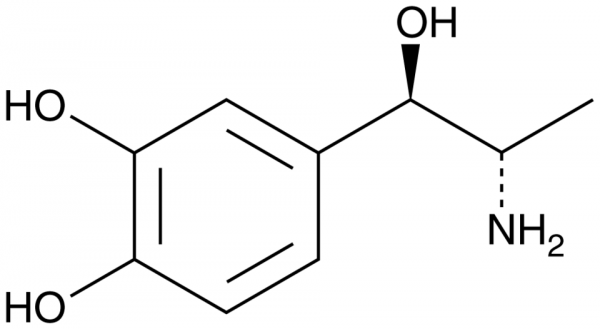
Levonordefrin is the (-)-enantiomer of nordefrin, a synthetic derivative of norepinephrine (Cay-16673), that acts as a vasoconstrictor. Levonordefrin (1-5 µg/kg) rapidly increases blood pressure and heart rate in anesthetized dogs and has a more potent effect on blood pressure than the dextronordefrin enantiomer. It also inhibits carbamylcholine-induced uterine contractions in isolated rat uterine strips (EC50 = 0.0032 µg/ml). Levonordefrin (0.514 and 1.542 mg/kg), in combination with mepivacaine (Cay-23402) and administered via intraoral infiltration, increases systolic arterial blood pressure in anesthetized dogs, however, the effect is not dose-dependent and the increase is not greater than 5.33%. Formulations containing levonordefrin have been used in combination with local anesthetics in dentistry to prolong anesthetic effects.Formal Name: 4-[(1R,2S)-2-amino-1-hydroxypropyl]-1,2-benzenediol. CAS Number: 829-74-3. Synonyms: Corbadrine, (-)-alpha-methyl Noradrenaline, (-)-3,4-dihydroxy Norephedrine. Molecular Formula: C9H13NO3. Formula Weight: 183.2. Purity: >95%. Formulation: (Request formulation change), A crystalline solid. Solubility: DMF: 30 mg/ml, DMSO: 30 mg/ml, Ethanol: 30 mg/ml, PBS (pH 7.2): 5 mg/ml. SMILES: OC1=C(O)C=CC([C@@H](O)[C@@H](N)C)=C1. InChi Code: InChI=1S/C9H13NO3/c1-5(10)9(13)6-2-3-7(11)8(12)4-6/h2-5,9,11-13H,10H2,1H3/t5-,9-/m0/s1. InChi Key: GEFQWZLICWMTKF-CDUCUWFYSA-N.
| Keywords: | (-)-3,4-dihydroxy Norephedrine, (-)-alpha-methyl Noradrenaline, Corbadrine, 4-[(1R,2S)-2-amino-1-hydroxypropyl]-1,2-benzenediol |
| Supplier: | Cayman Chemical |
| Supplier-Nr: | 23993 |
Properties
| Application: | Vasoconstrictor, alpha2-Adrenergic receptor agonist |
| MW: | 183.2 D |
| Formula: | C9H13NO3 |
| Purity: | >95% |
| Format: | Crystalline Solid |
Database Information
| CAS : | 829-74-3| Matching products |
| KEGG ID : | K04139 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +20°C (International: -20°C) |
| Signal Word: | Warning |
| GHS Hazard Pictograms: |
|
| H Phrases: | H302, H312, H315, H319, H332, H335 |
| P Phrases: | P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P361+364, P362+364, P403+233, P405, P501 |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed

