Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Laminins, a family of extracellular matrix glycoproteins, are the major noncollagenous... more
Product information "Anti-Laminin beta1"
Laminins, a family of extracellular matrix glycoproteins, are the major noncollagenous constituent of basement membranes. They have been implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signaling, neurite outgrowth and metastasis. Laminins are composed of 3 non identical chains: laminin alpha, beta and gamma (formerly A, B1, and B2, respectively) and they form a cruciform structure consisting of 3 short arms, each formed by a different chain, and a long arm composed of all 3 chains. Each laminin chain is a multidomain protein encoded by a distinct gene. Several isoforms of each chain have been described. This gene encodes the beta chain isoform laminin, beta 1. The beta 1 chain has 7 structurally distinct domains which it shares with other beta chain isomers. The C-terminal helical region containing domains I and II are separated by domain alpha, domains III and V contain several EGF-like repeats, and domains IV and VI have a globular conformation. Lam Protein function: Binding to cells via a high affinity receptor, laminin is thought to mediate the attachment, migration and organization of cells into tissues during embryonic development by interacting with other extracellular matrix components. Involved in the organization of the laminar architecture of cerebral cortex. It is probably required for the integrity of the basement membrane/glia limitans that serves as an anchor point for the endfeet of radial glial cells and as a physical barrier to migrating neurons. Radial glial cells play a central role in cerebral cortical development, where they act both as the proliferative unit of the cerebral cortex and a scaffold for neurons migrating toward the pial surface. [The UniProt Consortium]
| Keywords: | Anti-LAMB1, Anti-Laminin B1 chain, Anti-Laminin-8 subunit beta, Anti-Laminin subunit beta-1, Anti-Laminin-2 subunit beta, Anti-Laminin-1 subunit beta, Anti-Laminin-6 subunit beta, Anti-Laminin-10 subunit beta, Anti-Laminin-12 subunit beta, Laminin beta1 P |
| Supplier: | Elabscience |
| Supplier-Nr: | E-AB-70329 |
Properties
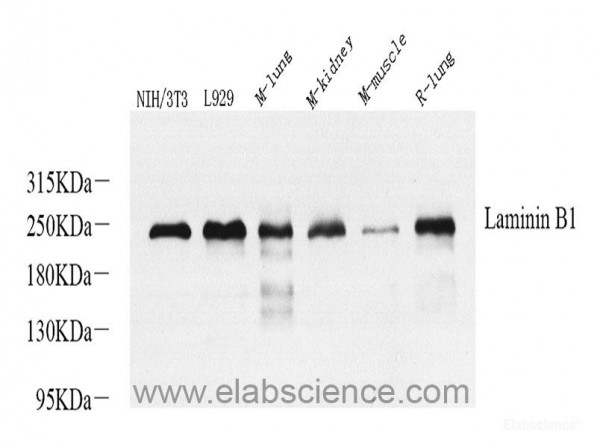
| Application: | WB |
| Antibody Type: | Polyclonal |
| Conjugate: | No |
| Host: | Rabbit |
| Species reactivity: | mouse, rat |
| Immunogen: | Recombinant protein corresponding to Mouse Laminin ß1 |
Database Information
| KEGG ID : | K05636 | Matching products |
| UniProt ID : | P07942 | Matching products |
| Gene ID : | GeneID 3912 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | 4°C (International: -20°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed




