Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
HSPD1,also known as HSP60,belongs to the chaperonin family and acts as a chaperone to enhance... more
Product information "Anti-HSP60"
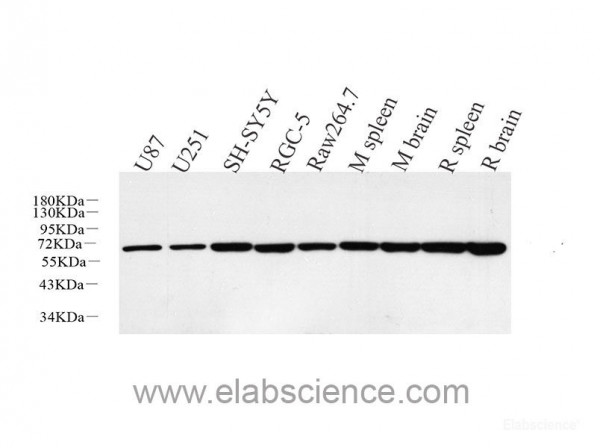
HSPD1,also known as HSP60,belongs to the chaperonin family and acts as a chaperone to enhance cell survival under physiological stresses. Hsp60 has been shown to be connected with many aspects of cell functions such as protein folding and assembling of polypeptide chains in mitochondria. Recently it has been reported that HSP60 is associated with apoptosis or inhibition of cancer cell growth. (21822415) Protein function: Chaperonin implicated in mitochondrial protein import and macromolecular assembly. Together with Hsp10, facilitates the correct folding of imported proteins. May also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix (PubMed:1346131, PubMed:11422376). The functional units of these chaperonins consist of heptameric rings of the large subunit Hsp60, which function as a back-to-back double ring. In a cyclic reaction, Hsp60 ring complexes bind one unfolded substrate protein per ring, followed by the binding of ATP and association with 2 heptameric rings of the co-chaperonin Hsp10. This leads to sequestration of the substrate protein in the inner cavity of Hsp60 where, for a certain period of time, it can fold undisturbed by other cell components. Synchronous hydrolysis of ATP in all Hsp60 subunits results in the dissociation of the chaperonin rings and the release of ADP and the folded substrate protein (Probable). [The UniProt Consortium]
| Keywords: | Anti-CPN60, Anti-Hsp60, Anti-HSPD1, Anti-HSP60, Anti-HSP-60, Anti-HuCHA60, EC=3.6.4.9, Anti-Chaperonin 60, Anti-60 kDa chaperonin, Anti-Heat shock protein 60, Anti-P60 lymphocyte protein, Anti-Mitochondrial matrix protein P1, HSP60 Polyclonal Antibody |
| Supplier: | Elabscience |
| Supplier-Nr: | E-AB-70103 |
Properties
| Application: | WB, IHC |
| Antibody Type: | Polyclonal |
| Conjugate: | No |
| Host: | Rabbit |
| Species reactivity: | human, mouse, rat |
| Immunogen: | KLH conjugated Synthetic peptide corresponding to Mouse HSP60 |
Database Information
| KEGG ID : | K04077 | Matching products |
| UniProt ID : | P10809 | Matching products |
| Gene ID : | GeneID 3329 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | 4°C (International: -20°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed