Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| ABE-35-1759-50 | 50 µl | - |
3 - 11 business days* |
574.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Casein kinases are operationally defined by their preferential utilization of acidic proteins... more
Product information "Anti-CK2 Alpha"
Casein kinases are operationally defined by their preferential utilization of acidic proteins such as caseins as substrates. The a and a' chains contain the catalytic site. Participates in Wnt signaling. CK2 phosphorylates 'Ser-392' of p53/TP53 following UV irradiation. Keller D.M., Zeng X., et al., Mol. Cell 7:283-292(2001) Keller D.M., Lu H. et al., J. Biol. Chem. 277:50206-50213(2002) Trembley J.H., Tatsumi S., et al., Mol. Cell. Biol. 25:1446-1457(2005) Niefind K., Guerra B., et al., Acta Crystallogr. D 56:1680-1684(2000) Protein function: Catalytic subunit of a constitutively active serine/threonine-protein kinase complex that phosphorylates a large number of substrates containing acidic residues C-terminal to the phosphorylated serine or threonine (PubMed:11239457, PubMed:11704824, PubMed:16193064, PubMed:19188443, PubMed:20625391, PubMed:22406621, PubMed:24962073). Regulates numerous cellular processes, such as cell cycle progression, apoptosis and transcription, as well as viral infection (PubMed:12631575, PubMed:19387552, PubMed:19387551). May act as a regulatory node which integrates and coordinates numerous signals leading to an appropriate cellular response (PubMed:12631575, PubMed:19387552, PubMed:19387551). During mitosis, functions as a component of the p53/TP53-dependent spindle assembly checkpoint (SAC) that maintains cyclin-B-CDK1 activity and G2 arrest in response to spindle damage (PubMed:11704824, PubMed:19188443). Also required for p53/TP53-mediated apoptosis, phosphorylating 'Ser-392' of p53/TP53 following UV irradiation. Can also negatively regulate apoptosis (PubMed:11239457). Phosphorylates the caspases CASP9 and CASP2 and the apoptotic regulator NOL3 (PubMed:16193064). Phosphorylation protects CASP9 from cleavage and activation by CASP8, and inhibits the dimerization of CASP2 and activation of CASP8 (PubMed:16193064). Regulates transcription by direct phosphorylation of RNA polymerases I, II, III and IV. Also phosphorylates and regulates numerous transcription factors including NF-kappa-B, STAT1, CREB1, IRF1, IRF2, ATF1, ATF4, SRF, MAX, JUN, FOS, MYC and MYB (PubMed:19387550, PubMed:12631575, PubMed:19387552, PubMed:19387551, PubMed:23123191). Phosphorylates Hsp90 and its co-chaperones FKBP4 and CDC37, which is essential for chaperone function (PubMed:19387550). Mediates sequential phosphorylation of FNIP1, promoting its gradual interaction with Hsp90, leading to activate both kinase and non-kinase client proteins of Hsp90 (PubMed:30699359). Regulates Wnt signaling by phosphorylating CTNNB1 and the transcription factor LEF1 (PubMed:19387549). Acts as an ectokinase that phosphorylates several extracellular proteins (PubMed:19387550, PubMed:12631575, PubMed:19387552, PubMed:19387551). During viral infection, phosphorylates various proteins involved in the viral life cycles of EBV, HSV, HBV, HCV, HIV, CMV and HPV (PubMed:19387550, PubMed:12631575, PubMed:19387552, PubMed:19387551). Phosphorylates PML at 'Ser-565' and primes it for ubiquitin-mediated degradation (PubMed:20625391, PubMed:22406621). Plays an important role in the circadian clock function by phosphorylating ARNTL/BMAL1 at 'Ser- 90' which is pivotal for its interaction with CLOCK and which controls CLOCK nuclear entry. Phosphorylates CCAR2 at 'Thr-454' in gastric carcinoma tissue (PubMed:24962073). [The UniProt Consortium]
| Keywords: | Anti-CK2A1, Anti-CSNK2A1, Anti-CK II alpha, Anti-Casein kinase II subunit alpha, Polyclonal Antibody to CK2 Alpha |
| Supplier: | Abeomics |
| Supplier-Nr: | 35-1759-50 |
Properties
| Application: | WB |
| Antibody Type: | Polyclonal |
| Conjugate: | No |
| Host: | Rabbit |
| Species reactivity: | human, mouse, rat |
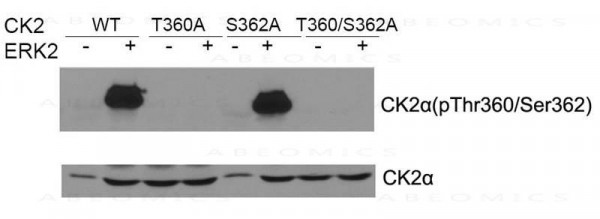
| Immunogen: | Peptide sequence around aa. 353~357 (S-G-I-S-S) derived from Human CK2a. |
| Format: | Purified |
Database Information
| KEGG ID : | K03097 | Matching products |
| UniProt ID : | P68400 | Matching products |
| Gene ID : | GeneID 1457 | Matching products |
Handling & Safety
| Storage: | +4°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed





