Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Cenicriviroc is an orally bioavailable antagonist of C-C chemokine receptor type 5 (CCR5) and... more
Product information "Cenicriviroc"
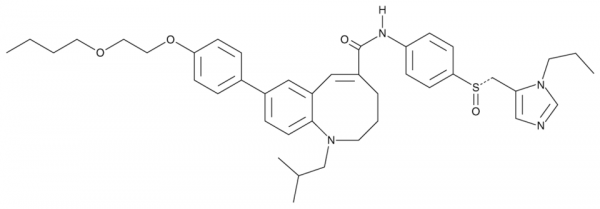
Cenicriviroc is an orally bioavailable antagonist of C-C chemokine receptor type 5 (CCR5) and CCR2 that inhibits binding of macrophage inflammatory protein 1alpha (MIP-1alpha, IC50 = 2.3 nM) and monocyte chemotactic protein 1 (MCP-1, IC50 = 5.9 nM), respectively, in CHO cells. Cenicriviroc is also an antagonist of CCR3 and CCR4 (IC50s = 2.4 and 1.1 µM, respectively), however, it does not inhibit agonist binding to CCR1 or CCR7 at concentrations up to 10 µM. At a concentration of 100 nM, cenicriviroc completely inhibits replication of R5 HIV-1 in U87.CD4.CCR5 cells. Cenicriviroc inhibits replication of the R5 HIV-1 strains JR-FL and KK in peripheral blood mononuclear cells (PBMCs) with EC50 values ranging from 21 to 210 pM and 33 to 91 pM, respectively. In vivo, cenicriviroc (20 mg/kg per day) reduces collagen deposition, levels of collagen type 1 protein and mRNA expression, and non-alcoholic fatty liver disease activity score in a mouse model of non-alcoholic steatohepatitis (NASH). It also reduces collagen type 1 protein levels, mRNA expression, and collagen deposition in a mouse model of unilateral ureter obstruction and a rat model of thioacetamide-induced liver fibrosis.Formal Name: 8-[4-(2-butoxyethoxy)phenyl]-1,2,3,4-tetrahydro-1-(2-methylpropyl)-N-[4-[(S)-[(1-propyl-1H-imidazol-5-yl)methyl]sulfinyl]phenyl]-1-benzazocine-5-carboxamide. CAS Number: 497223-25-3. Synonyms: TAK-652. Molecular Formula: C41H52N4O4S. Formula Weight: 696.9. Purity: >95%. Formulation: (Request formulation change), A crystalline solid. Solubility: DMF: 20 mg/ml, DMSO: 20 mg/ml, DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml, Ethanol: 5 mg/ml. lambdamax: 295 nm. SMILES: CCCCOCCOC1=CC=C(C2=CC=C(N(CC(C)C)CCC/C(C(NC3=CC=C([S@](CC4=CN=CN4CCC)=O)C=C3)=O)=C\5)C5=C2)C=C1. InChi Code: InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/b34-26+/t50-/m0/s1. InChi Key: PNDKCRDVVKJPKG-WHERJAGFSA-N.
| Keywords: | TAK-652, 8-[4-(2-butoxyethoxy)phenyl]-1,2,3,4-tetrahydro-1-(2-methylpropyl)-N-[4-[(S)-[(1-propyl-1H-imidazol-5-yl)methyl]sulfinyl]phenyl]-1-benzazocine-5-carboxamide |
| Supplier: | Cayman Chemical |
| Supplier-Nr: | 23927 |
Properties
| Application: | Antiviral, CCR5 / CCR2 antagonist |
| MW: | 696.9 D |
| Formula: | C41H52N4O4S |
| Purity: | >95% |
| Format: | Crystalline Solid |
Database Information
| CAS : | 497223-25-3| Matching products |
| KEGG ID : | K04180 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | -20°C (International: -20°C) |
| Signal Word: | Warning |
| GHS Hazard Pictograms: |
|
| H Phrases: | H302, H315, H319, H335 |
| P Phrases: | P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362+364, P403+233, P405, P501 |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed