Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| NSJ-R31887 | 100 µg | - | - |
3 - 10 business days* |
755.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
0.5mg/ml if reconstituted with 0.2ml sterile DI water. CYP1A1 is involved in phase I xenobiotic... more
Product information "Anti-CYP1A1"
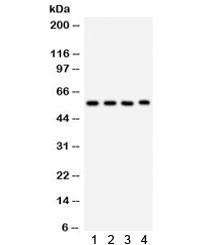
0.5mg/ml if reconstituted with 0.2ml sterile DI water. CYP1A1 is involved in phase I xenobiotic and drug metabolism (one substrate of it is theophylline). It is inhibited by fluoroquinolones and macrolides and induced by aromatic hydrocarbons. CYP1A1 is also known as AHH (aryl hydrocarbon hydroxylase). It is involved in the metabolic activation of aromatic hydrocarbons (polycyclic aromatic hydrocarbons, PAH), for example, benzo(a)pyrene (BP), by transforming it to an epoxide. In this reaction, the oxidation of benzo[a]pyrene is catalysed by CYP1A1 to form BP-7,8-epoxide, which can be further oxidized by epoxide hydrolase (EH) to form BP-7,8-dihydrodiol. Finally CYP1A1 catalyses this intermediate to form BP-7,8-dihydrodiol-9,10-epoxide, which is the ultimate carcinogen. However, an in vivo experiment with gene-deficient mice has found that the hydroxylation of benzo(a)pyrene by CYP1A1 can have an overall protective effect on the DNA, rather than contributing to potentially carcinogenic DNA modifications. This effect is likely due to the fact that CYP1A1 is highly active in the intestinal mucosa, and thus inhibits infiltration of ingested benzo(a)pyrene carcinogen into the systemic circulation. Protein function: A cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins (PubMed:11555828, PubMed:14559847, PubMed:12865317, PubMed:15805301, PubMed:15041462, PubMed:18577768, PubMed:19965576, PubMed:20972997, PubMed:10681376). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (NADPH--hemoprotein reductase) (PubMed:11555828, PubMed:14559847, PubMed:12865317, PubMed:15805301, PubMed:15041462, PubMed:18577768, PubMed:19965576, PubMed:20972997, PubMed:10681376). Catalyzes the hydroxylation of carbon-hydrogen bonds. Exhibits high catalytic activity for the formation of hydroxyestrogens from estrone (E1) and 17beta-estradiol (E2), namely 2-hydroxy E1 and E2, as well as D-ring hydroxylated E1 and E2 at the C15-alpha and C16- alpha positions (PubMed:11555828, PubMed:14559847, PubMed:12865317, PubMed:15805301). Displays different regioselectivities for polyunsaturated fatty acids (PUFA) hydroxylation (PubMed:15041462, PubMed:18577768). Catalyzes the epoxidation of double bonds of certain PUFA (PubMed:15041462, PubMed:19965576, PubMed:20972997). Converts arachidonic acid toward epoxyeicosatrienoic acid (EET) regioisomers, 8,9-, 11,12-, and 14,15-EET, that function as lipid mediators in the vascular system (PubMed:20972997). Displays an absolute stereoselectivity in the epoxidation of eicosapentaenoic acid (EPA) producing the 17(R),18(S) enantiomer (PubMed:15041462). May play an important role in all-trans retinoic acid biosynthesis in extrahepatic tissues. Catalyzes two successive oxidative transformation of all-trans retinol to all-trans retinal and then to the active form all-trans retinoic acid (PubMed:10681376). May also participate in eicosanoids metabolism by converting hydroperoxide species into oxo metabolites (lipoxygenase-like reaction, NADPH-independent) (PubMed:21068195). [The UniProt Consortium]
| Keywords: | Anti-CYPIA1, Anti-Cytochrome P450-C, Anti-Cytochrome P450-P1, Anti-Cytochrome P450 1A1, Anti-Cytochrome P450 form 6, Anti-Hydroperoxy icosatetraenoate dehydratase, CYP1A1 Antibody |
| Supplier: | NSJ Bioreagents |
| Supplier-Nr: | R31887 |
Properties
| Application: | WB, IHC (paraffin), (IF), FC, IF |
| Antibody Type: | Polyclonal |
| Conjugate: | No |
| Host: | Rabbit |
| Species reactivity: | human, mouse, rat |
| Immunogen: | Amino acids 183-320 of human CYP1A1 |
| Format: | Purified |
Database Information
| KEGG ID : | K07408 | Matching products |
| UniProt ID : | P04798 | Matching products |
| Gene ID : | GeneID 1543 | Matching products |
Handling & Safety
| Storage: | +4°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed