Cookie-Einstellungen
Diese Website benutzt Cookies, die für den technischen Betrieb der Website erforderlich sind und stets gesetzt werden. Andere Cookies, die den Komfort bei Benutzung dieser Website erhöhen, der Direktwerbung dienen oder die Interaktion mit anderen Websites und sozialen Netzwerken vereinfachen sollen, werden nur mit Ihrer Zustimmung gesetzt.
Konfiguration
Technisch erforderlich
Diese Cookies sind für die Grundfunktionen des Shops notwendig.
"Alle Cookies ablehnen" Cookie
"Alle Cookies annehmen" Cookie
Ausgewählter Shop
CSRF-Token
Cookie-Einstellungen
FACT-Finder Tracking
Individuelle Preise
Kundenspezifisches Caching
Session
Währungswechsel
Komfortfunktionen
Diese Cookies werden genutzt um das Einkaufserlebnis noch ansprechender zu gestalten, beispielsweise für die Wiedererkennung des Besuchers.
Facebook-Seite in der rechten Blog - Sidebar anzeigen
Merkzettel
Statistik & Tracking
Endgeräteerkennung
Kauf- und Surfverhalten mit Google Tag Manager
Partnerprogramm
Bei Fragen nutzen Sie gerne unser Kontaktformular.
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Organism: Homo sapiens (Human). Source: E.coli. Expression Region: 25-293aa. Protein Length:... mehr
Produktinformationen "Endoplasmic reticulum chaperone BiP (HSPA5), partial, human, recombinant"
Organism: Homo sapiens (Human). Source: E.coli. Expression Region: 25-293aa. Protein Length: Partial. Tag Info: N-terminal 6xHis-tagged. Target Protein Sequence: EDVGTVVGID LGTTYSCVGV FKNGRVEIIA NDQGNRITPS YVAFTPEGER LIGDAAKNQL TSNPENTVFD AKRLIGRTWN DPSVQQDIKF LPFKVVEKKT KPYIQVDIGG GQTKTFAPEE ISAMVLTKMK ETAEAYLGKK VTHAVVTVPA YFNDAQRQAT KDAGTIAGLN VMRIINEPTA AAIAYGLDKR EGEKNILVFD LGGGTFDVSL LTIDNGVFEV VATNGDTHLG GEDFDQRVME HFIKLYKKKT GKDVRKDNRA VQKLRREVE. Purity: Greater than 90% as determined by SDS-PAGE. Endotoxin: Not test. Biological Activity: n/a. Form: Liquid or Lyophilized powder. Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0. Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20 °C/-80 °C. Our default final concentration of glycerol is 50%. Customers could use it as reference. Storage: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20 °C/-80 °C. The shelf life of lyophilized form is 12 months at -20 °C/-80 °C. Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4 °C for up to one week. Relevance: Probably plays a role in facilitating the assbly of multimeric protein complexes inside the endoplasmic reticulum. Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10, probably to facilitate the release of DNAJC10 from its substrate. Reference: Chao C.C.K. Grp78 is involved in the quality control of the LDL-receptor.Hansen J.J., Nielsen M.N., Jorgensen M.M., Gregersen N., Bolund L. Sequence differences between human grp78/BiP isolated from HeLa cells and previously reported human sequences.Bermudez-Fajardo A., Llewellyn D.H., Campbell A.K., Errington R.R.NIEHS SNPs programDNA sequence and analysis of human chromosome 9.Humphray S.J., Oliver K., Hunt A.R., Plumb R.W., Loveland J.E., Howe K.L., Andrews T.D., Searle S., Hunt S.E., Scott C.E., Jones M.C., Ainscough R., Almeida J.P., Ambrose K.D., Ashwell R.I.S., Babbage A.K., Babbage S., Bagguley C.L. , Bailey J., Banerjee R., Barker D.J., Barlow K.F., Bates K., Beasley H., Beasley O., Bird C.P., Bray-Allen S., Brown A.J., Brown J.Y., Burford D., Burrill W., Burton J., Carder C., Carter N.P., Chapman J.C., Chen Y., Clarke G., Clark S.Y., Clee C.M., Clegg S., Collier R.E., Corby N., Crosier M., Cummings A.T., Davies J., Dhami P., Dunn M., Dutta I., Dyer L.W., Earthrowl M.E., Faulkner L., Fleming C.J., Frankish A., Frankland J.A., French L., Fricker D.G., Garner P., Garnett J., Ghori J., Gilbert J.G.R., Glison C., Grafham D.V., Gribble S., Griffiths C., Griffiths-Jones S., Grocock R., Guy J., Hall R.E., Hammond S., Harley J.L., Harrison E.S.I., Hart E.A., Heath P.D., Henderson C.D., Hopkins B.L., Howard P.J., Howden P.J., Huckle E., Johnson C., Johnson D., Joy A.A., Kay M., Keenan S., Kershaw J.K., Kimberley A.M., King A., Knights A., Laird G.K., Langford C., Lawlor S., Leongamornlert D.A., Leversha M., Lloyd C., Lloyd D.M., Lovell J., Martin S., Mashreghi-Mohammadi M., Matthews L., McLaren S., McLay K.E., McMurray A., Milne S., Nickerson T., Nisbett J., Nordsiek G., Pearce A.V., Peck A.I., Porter K.M., Pandian R., Pelan S., Phillimore B., Povey S., Ramsey Y., Rand V., Scharfe M., Sehra H.K., Shownkeen R., Sims S.K., Skuce C.D., Smith M., Steward C.A., Swarbreck D., Sycamore N., Tester J., Thorpe A., Tracey A., Tromans A., Thomas D.W., Wall M., Wallis J.M., West A.P., Whitehead S.L., Willey D.L., Williams S.A., Wilming L., Wray P.W., Young L., Ashurst J.L., Coulson A., Blocker H., Durbin R.M., Sulston J.E., Hubbard T., Jackson M.J., Bentley D.R., Beck S., Rogers J., Dunham I.Nature 429:369-374(2004). Function: Plays a role in facilitating the assembly of multimeric protein complexes inside the endoplasmic reticulum. Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10, probably to facilitate the release of DNAJC10 from its substrate (By similarity).
| Schlagworte: | BiP, GRP-78, HSP70 family protein 5, Binding-immunoglobulin protein, 78 kDa glucose-regulated protein, Endoplasmic reticulum chaperone BiP, Heat shock protein family A member 5, Heat shock protein 70 family protein 5, Recombinant Human Endoplasmic reticul |
| Hersteller: | Cusabio |
| Hersteller-Nr: | EP010827HU1 |
Eigenschaften
| Anwendung: | Activity not tested |
| Konjugat: | No |
| Wirt: | E.coli |
| Spezies-Reaktivität: | human |
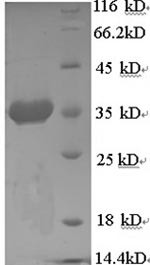
| MW: | 33.6 kD |
| Reinheit: | >90% (SDS-PAGE) |
Datenbank Information
| KEGG ID : | K09490 | Passende Produkte |
| UniProt ID : | P11021 | Passende Produkte |
| Gene ID : | GeneID 3309 | Passende Produkte |
Handhabung & Sicherheit
| Lagerung: | -20°C |
| Versand: | +4°C (International: +4°C) |
Achtung
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Hier kriegen Sie ein Zertifikat
Loggen Sie sich ein oder registrieren Sie sich, um Analysenzertifikate anzufordern.
Bewertungen lesen, schreiben und diskutieren... mehr
Kundenbewertungen für "Endoplasmic reticulum chaperone BiP (HSPA5), partial, human, recombinant"
Bewertung schreiben
Loggen Sie sich ein oder registrieren Sie sich, um eine Produktbewertung abzugeben.
Zuletzt angesehen

