Bei Fragen nutzen Sie gerne unser Kontaktformular.
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Zuletzt angesehen
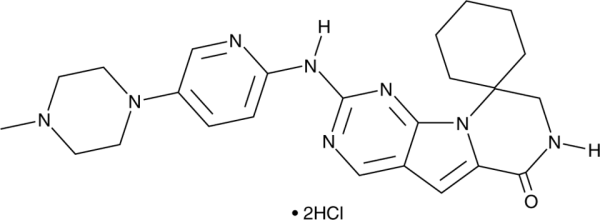
| Schlagworte: | G1T28, 7',8'-dihydro-2'-[[5-(4-methyl-1-piperazinyl)-2-pyridinyl]amino]-spiro[cyclohexane-1,9'(6'H)-pyrazino[1',2':1,5]pyrrolo[2,3-d]pyrimidin]-6'-one, dihydrochloride |
| Hersteller: | Cayman Chemical |
| Hersteller-Nr: | 37297 |
Eigenschaften
| Anwendung: | Cdk4 / Cdk6 inhibitor |
| MW: | 519.5 D |
| Formel: | C24H30N8O . 2HCl |
| Reinheit: | >98% |
| Format: | Solid |
Datenbank Information
| CAS : | 1977495-97-8| Passende Produkte |
| KEGG ID : | K02089 | Passende Produkte |
Handhabung & Sicherheit
| Lagerung: | -20°C |
| Versand: | +20°C (International: -20°C) |
