Cookie-Einstellungen
Diese Website benutzt Cookies, die für den technischen Betrieb der Website erforderlich sind und stets gesetzt werden. Andere Cookies, die den Komfort bei Benutzung dieser Website erhöhen, der Direktwerbung dienen oder die Interaktion mit anderen Websites und sozialen Netzwerken vereinfachen sollen, werden nur mit Ihrer Zustimmung gesetzt.
Konfiguration
Technisch erforderlich
Diese Cookies sind für die Grundfunktionen des Shops notwendig.
"Alle Cookies ablehnen" Cookie
"Alle Cookies annehmen" Cookie
Ausgewählter Shop
CSRF-Token
Cookie-Einstellungen
FACT-Finder Tracking
Individuelle Preise
Kundenspezifisches Caching
Session
Währungswechsel
Komfortfunktionen
Diese Cookies werden genutzt um das Einkaufserlebnis noch ansprechender zu gestalten, beispielsweise für die Wiedererkennung des Besuchers.
Facebook-Seite in der rechten Blog - Sidebar anzeigen
Merkzettel
Statistik & Tracking
Endgeräteerkennung
Kauf- und Surfverhalten mit Google Tag Manager
Partnerprogramm
Bei Fragen nutzen Sie gerne unser Kontaktformular.
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Sorafenib is a multi-kinase inhibitor that inhibits Raf-1 and B-RAF (IC50s = 6 and 22 µM,... mehr
Produktinformationen "Sorafenib (tosylate)"
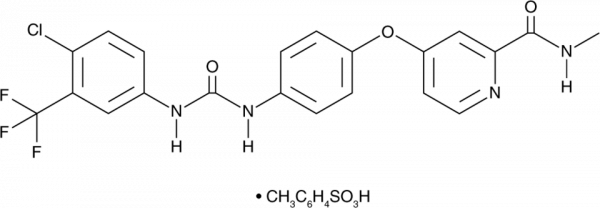
Sorafenib is a multi-kinase inhibitor that inhibits Raf-1 and B-RAF (IC50s = 6 and 22 µM, respectively), as well as the receptor tyrosine kinases VEGFR2, VEGFR3, PDGFRbeta, FLT3, and c-Kit (IC50s = 90, 15, 20, 57, and 58 nM, respectively). It is selective for these kinases over 12 other kinases, including ERK1, MEK1, EGFR, and HER2 (IC50s = >10 µM for all). Sorafenib inhibits proliferation of PLC/PRF/5 and HepG2 cells (IC50s = 6.3 and 4.5 µM, respectively) and induces apoptosis in these cells. It completely inhibits tumor growth in a PLC/PRF/5 mouse xenograft model when administered at a dose of 30 mg/kg and reduces basic FGF-induced angiogenesis in a Matrigel(TM) assay in vivo. Sorafenib (10 µM) induces ferroptotic cell death in HT-1080 fibrosarcoma cells, an effect that can be blocked by the ferroptosis inhibitors ferrostatin-1 (Cay-17729), deferoxamine (Cay-14595), and beta-mercaptoethanol, but does not induce ferroptosis in a variety of other cancer cell lines. It inhibits glutamate release by the system xc- cystine/glutamate transporter in HT-1080 cells when used at concentrations ranging from 2.5 to 10 µM, decreases glutathione levels, and increases lipid peroxidation. Sorafenib also inhibits replication of hepatitis C virus (HCV) in Huh7.5 cells (IC50 = 7.2 µM). Formulations containing sorafenib have been used in the treatment of hepatocellular, renal cell, and thyroid carcinomas.Formal Name: 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide, 4-methylbenzenesulfonate. CAS Number: 475207-59-1. Synonyms: BAY 43-9006 mono-p-tosylate, BAY 54-9085. Molecular Formula: C21H16ClF3N4O3 . C7H8O3S. Formula Weight: 637.0. Purity: >98%. Formulation: (Request formulation change), A solid. Solubility: DMF: 3 mg/ml, DMSO: 5 mg/ml, Ethanol: insol, PBS (pH 7.2): insol. lambdamax: 266 nm. SMILES: O=C(NC1=CC(C(F)(F)F)=C(Cl)C=C1)NC2=CC=C(OC3=CC=NC(C(NC)=O)=C3)C=C2.O=S(O)(C4=CC=C(C)C=C4)=O. InChi Code: InChI=1S/C21H16ClF3N4O3.C7H8O3S/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25,1-6-2-4-7(5-3-6)11(8,9)10/h2-11H,1H3,(H,26,30)(H2,28,29,31),2-5H,1H3,(H,8,9,10). InChi Key: IVDHYUQIDRJSTI-UHFFFAOYSA-N.
| Schlagworte: | BAY 43-9006 mono-p-tosylate, BAY 54-9085, 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide, 4-methylbenzenesulfonate |
| Hersteller: | Cayman Chemical |
| Hersteller-Nr: | 35612 |
Eigenschaften
| Anwendung: | Multi-kinase inhibitor |
| MW: | 637 D |
| Formel: | C21H16ClF3N4O3 . C7H8O3S |
| Reinheit: | >98% |
| Format: | Solid |
Datenbank Information
| CAS : | 475207-59-1| Passende Produkte |
| KEGG ID : | K05089 | Passende Produkte |
Handhabung & Sicherheit
| Lagerung: | -20°C |
| Versand: | +20°C (International: -20°C) |
| Signalwort: | Danger |
| GHS-Piktogramme: |
|
| H-Sätze: | H302, H332, H360, H362, H373, H412, H402 |
| P-Sätze: | P201, P202, P260, P261, P263, P264, P270, P271, P273, P280, P312, P314, P330, P301+P310, P304+P340, P308+P313, P405, P501 |
Achtung
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Hier folgen Informationen zur Produktreferenz.
mehr
Hier kriegen Sie ein Zertifikat
Loggen Sie sich ein oder registrieren Sie sich, um Analysenzertifikate anzufordern.
Bewertungen lesen, schreiben und diskutieren... mehr
Kundenbewertungen für "Sorafenib (tosylate)"
Bewertung schreiben
Loggen Sie sich ein oder registrieren Sie sich, um eine Produktbewertung abzugeben.
Zuletzt angesehen

