Cookie-Einstellungen
Diese Website benutzt Cookies, die für den technischen Betrieb der Website erforderlich sind und stets gesetzt werden. Andere Cookies, die den Komfort bei Benutzung dieser Website erhöhen, der Direktwerbung dienen oder die Interaktion mit anderen Websites und sozialen Netzwerken vereinfachen sollen, werden nur mit Ihrer Zustimmung gesetzt.
Konfiguration
Technisch erforderlich
Diese Cookies sind für die Grundfunktionen des Shops notwendig.
"Alle Cookies ablehnen" Cookie
"Alle Cookies annehmen" Cookie
Ausgewählter Shop
CSRF-Token
Cookie-Einstellungen
FACT-Finder Tracking
Individuelle Preise
Kundenspezifisches Caching
Session
Währungswechsel
Komfortfunktionen
Diese Cookies werden genutzt um das Einkaufserlebnis noch ansprechender zu gestalten, beispielsweise für die Wiedererkennung des Besuchers.
Facebook-Seite in der rechten Blog - Sidebar anzeigen
Merkzettel
Statistik & Tracking
Endgeräteerkennung
Kauf- und Surfverhalten mit Google Tag Manager
Partnerprogramm
Bei Fragen nutzen Sie gerne unser Kontaktformular.
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Protein function: Serine/threonine-protein kinase that acts as a molecular sensor for DNA damage.... mehr
Produktinformationen "Anti-PRKDC"
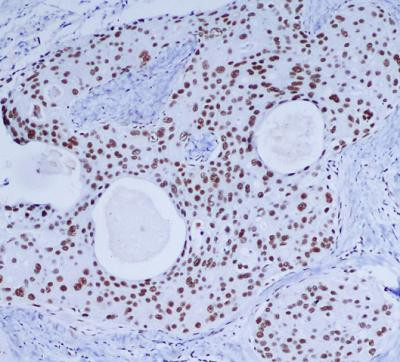
Protein function: Serine/threonine-protein kinase that acts as a molecular sensor for DNA damage. Involved in DNA non-homologous end joining (NHEJ) required for double-strand break (DSB) repair and V(D)J recombination (PubMed:11955432, PubMed:12649176, PubMed:14734805). Must be bound to DNA to express its catalytic properties. Promotes processing of hairpin DNA structures in V(D)J recombination by activation of the hairpin endonuclease artemis (DCLRE1C) (PubMed:11955432). The assembly of the DNA-PK complex at DNA ends is also required for the NHEJ ligation step (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). Required to protect and align broken ends of DNA (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). May also act as a scaffold protein to aid the localization of DNA repair proteins to the site of damage (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). Found at the ends of chromosomes, suggesting a further role in the maintenance of telomeric stability and the prevention of chromosomal end fusion. Also involved in modulation of transcription (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). As part of the DNA-PK complex, involved in the early steps of ribosome assembly by promoting the processing of precursor rRNA into mature 18S rRNA in the small-subunit processome (PubMed:32103174). Binding to U3 small nucleolar RNA, recruits PRKDC and XRCC5/Ku86 to the small-subunit processome (PubMed:32103174). Recognizes the substrate consensus sequence [ST]-Q (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). Phosphorylates 'Ser-139' of histone variant H2AX, thereby regulating DNA damage response mechanism (PubMed:14627815, PubMed:16046194). Phosphorylates DCLRE1C, c-Abl/ABL1, histone H1, HSPCA, c-jun/JUN, p53/TP53, PARP1, POU2F1, DHX9, FH, SRF, XRCC1, XRCC1, XRCC4, XRCC5, XRCC6, WRN, MYC and RFA2 (PubMed:2507541, PubMed:2247066, PubMed:1597196, PubMed:8407951, PubMed:8464713, PubMed:9362500, PubMed:9139719, PubMed:10026262, PubMed:10467406, PubMed:12509254, PubMed:11889123, PubMed:14612514, PubMed:14704337, PubMed:16397295, PubMed:26237645, PubMed:28712728). Can phosphorylate C1D not only in the presence of linear DNA but also in the presence of supercoiled DNA (PubMed:9679063). Ability to phosphorylate p53/TP53 in the presence of supercoiled DNA is dependent on C1D (PubMed:9363941). Contributes to the determination of the circadian period length by antagonizing phosphorylation of CRY1 'Ser-588' and increasing CRY1 protein stability, most likely through an indirect mechanism. Plays a role in the regulation of DNA virus-mediated innate immune response by assembling into the HDP-RNP complex, a complex that serves as a platform for IRF3 phosphorylation and subsequent innate immune response activation through the cGAS-STING pathway (PubMed:28712728). [The UniProt Consortium]
| Schlagworte: | Anti-DNA-dependent protein kinase catalytic subunit, Anti-DNA-PK catalytic subunit, Anti-DNA-PKcs, Anti-DNPK1, Anti-p460, PRKDC Antibody |
| Hersteller: | Absea |
| Hersteller-Nr: | KC-6291 |
Eigenschaften
| Antikörper-Typ: | Monoclonal |
| Klon: | 2T25 |
| Konjugat: | No |
| Wirt: | Mouse |
| Spezies-Reaktivität: | human |
| Format: | Solution |
Datenbank Information
| KEGG ID : | K06642 | Passende Produkte |
| UniProt ID : | P78527 | Passende Produkte |
| Gene ID : | GeneID 5591 | Passende Produkte |
Handhabung & Sicherheit
| Lagerung: | -20°C (avoid repeat freezing and thawing cycles) |
| Versand: | +4°C (International: +4°C) |
Achtung
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Hier kriegen Sie ein Zertifikat
Loggen Sie sich ein oder registrieren Sie sich, um Analysenzertifikate anzufordern.
Bewertungen lesen, schreiben und diskutieren... mehr
Kundenbewertungen für "Anti-PRKDC"
Bewertung schreiben
Loggen Sie sich ein oder registrieren Sie sich, um eine Produktbewertung abzugeben.
Zuletzt angesehen